Ozone Exposure and Our Health
Ozone can be “good” or “bad” for us, depending on where it is found. This article outlines some of the health effects caused by ozone, what we are doing to reduce exposure now, and what can be done in future.
Jack Turner - Environmental Data Analyst - 21 October 2021

Ozone. The first thing that may spring to mind for most people is the ozone layer, over 20km up in the stratosphere, quietly absorbing harmful ultraviolet (UV) radiation emitted from the sun.
Exposure to UV radiation is one of the main causes of skin cancer. Although the ozone layer provides humanity with some protection against this disease, this author reminds you to wear sun protection to protect your skin!
The ozone layer's protection against UV radiation also prevents the heating of the Earth's surface, a phenomenon that is closely linked to climate change.
The depletion of the ozone layer has been a subject of hot debate. This is because:
- Less ozone means less protection against UV radiation and therefore more skin cancer.
- Less ozone available to break down UV radiation also leads to a greater heating of the Earth's surface, accelerating climate change.
From the above, could assume that ozone is good for us, right?
Well, it is more complicated than that. Ozone in the stratosphere is a good thing. Though, ozone at ground level, where we can breathe it in, is a nasty gas.
What is ozone and how does it form?
Ozone is a highly reactive gas comprising of three oxygen molecules, and therefore has the chemical formula O3. In the stratosphere, it is formed through naturally occurring reactions between oxygen molecules, aided by UV radiation. At ground level, ozone is typically formed from reactions between volatile organic compounds (VOCs) and nitrogen oxides, both of which are emitted from human sources such as combustion such as from car engines (nitrogen oxides) and chemical plants (VOCs).
VOCs can also be naturally emitted by plants as a by-product of photosynthesis. Somewhat confusingly, however, nitrogen oxides can also react with ozone and reduce concentrations at ground level. This is sometimes observed at roadside locations with high traffic counts which may have lower ozone levels than areas further from the road.
The reactions that take place to form street-level ozone are also dependent on temperature and light. So street-level ozone levels tend to be higher on hot, sunny days in the summer. On a shorter timescale, ozone levels tend to be highest in the afternoon when solar radiation is at its strongest.
What are the health impacts of street-level ozone?
Due to the highly reactive nature of ozone, most effects and symptoms of ozone exposure are found at the initial contact points: in the mouth, nose, airways and lungs. Lung function can decrease as cells and tissues within the lungs are damaged by ozone. Cells and tissue in the respiratory tract also get inflamed due to reactions with ozone.
Symptoms can express themselves in a variety of ways, including:
- a sore throat
- shortness of breath
- coughing
- discomfort when breathing in
There is also strong evidence to show that exposure to ozone can trigger asthma attacks and result in asthma symptoms being more severe. Some studies have also linked ozone exposure to increased sick days, increased hospital admissions and in severe cases, increased mortality. The US Environmental Protection Agency outline these effects in the pyramid shown below.
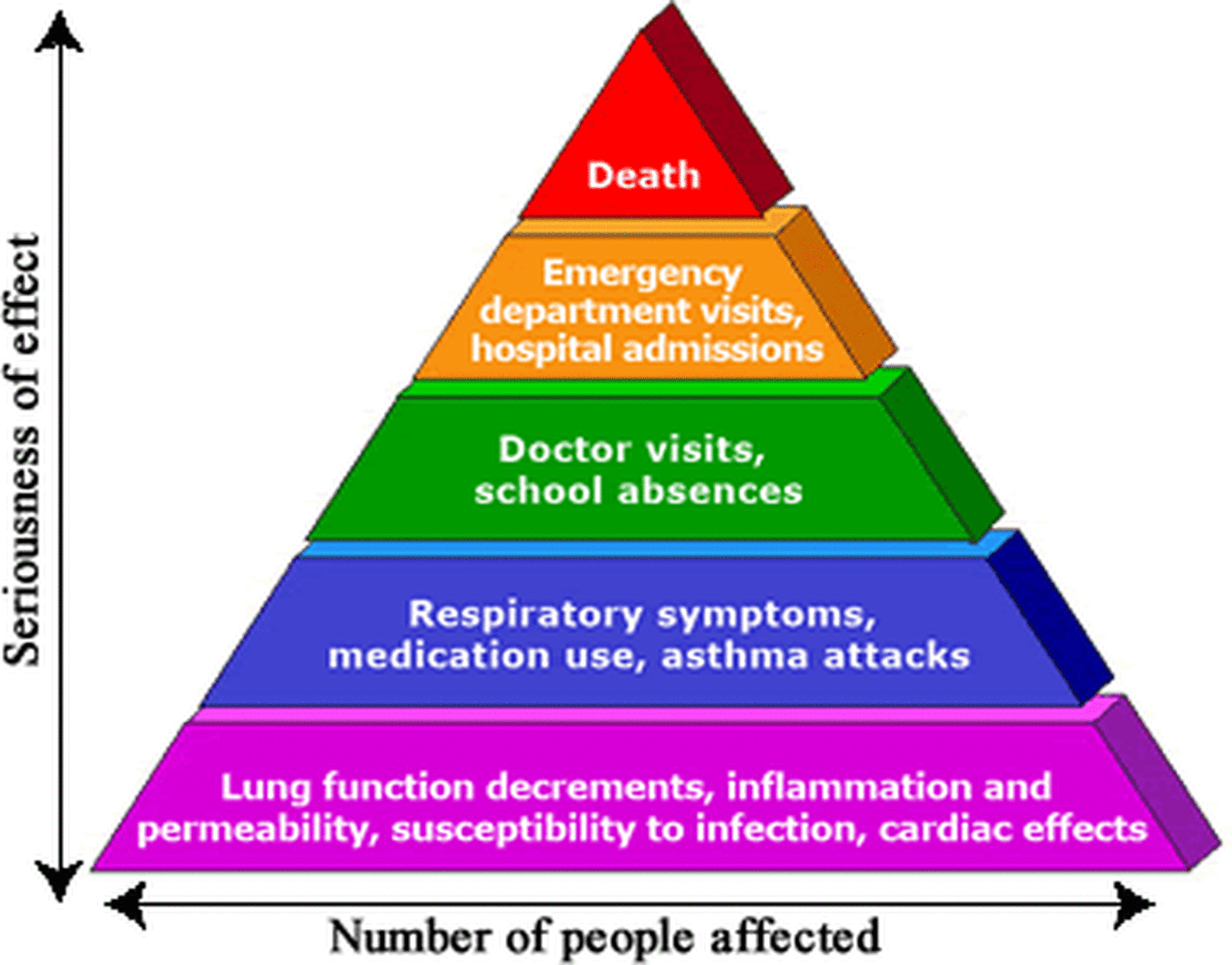
People most likely to be at the greatest risk of exposure to unsafe levels of ozone include people exercising outdoors (in this instance deeper inhalation can result in ozone getting further into the lungs); and people who work outdoors jobs.
Different people may respond differently when exposed to ozone; with some evidence that children and younger adults are more sensitive to mild-to-moderate impacts such as decreased lung function; however, hospitalisations and mortality relating to ozone exposure are more likely to be people over 65.
What can we do to reduce ozone exposure?
The World Health Organisation has suggested that ozone levels should not exceed 100µg/m3, but this value is often exceeded, especially during periods of warm, dry weather in spring and summer. It is difficult to reduce ozone concentrations themselves, as street-level ozone does not have one individual source, however, we can reduce the substances which react together to form ozone.
Nitrogen oxide concentrations can be reduced by reducing the number of cars on the roads, and by using cleaner non-combustion energy sources, while VOCs are harder to remove since they can be naturally occurring, but also because of a lack of monitoring and therefore understanding of them.
There is a complex relationship between nitrogen oxides and ozone, where nitrogen oxides react to both form ozone and break ozone down. This makes the overall picture unclear as to how clean air policies may affect ozone concentrations in urban areas. In the first nationwide COVID-19 lockdown, where fewer car journeys were made, and concentrations of most pollutants, including nitrogen oxides, decreased, ozone levels actually increased in some urban areas. This begs the question, are clean air strategies effective in reducing ozone concentrations?
Is traditional air quality monitoring enough?
Ozone is monitored in the UK as part of the Automatic Urban and Rural Network (AURN) which is the DEFRA approved monitoring stations for pollutants. As such, AURN sites are found in most large towns and cities, where pollution levels tend to be higher.
However, the complex reactions resulting in both the formation and breakdown of ozone suggest there may be hyperlocal variations in concentration not detected by traditional monitoring methods. Better monitoring of ozone can determine hotspots and create a rationale for new clean air policies; as well as being used to inform vulnerable residents when there is a high pollution episode to avoid being exposed to dangerous ozone levels.
At Vortex, we believe that to know where to act against air pollution, you must first get a better understanding of it. In this whitepaper, we use ozone pollution data from 19 AURN sites across London to demonstrate why hyperlocal monitoring is now essential. Read it here.
If you want to learn more about how Vortex helps to reduce air pollution, check out our VTX Air and get in touch.
More blogs

Should clean air be a national priority?
Millions across the UK are still exposed to harmful levels of air pollution every day. As we mark Clean Air Day, it’s time to ask: if clean air impacts our health, our…

Engaging communities for cleaner air
As we mark Clean Air Day, we proudly highlight the significance of engagement and behaviour change to make a tangible difference in the fight against air pollution.